Concurrently, a very different theory evolved for understanding the electronic structure of metallic solids (see for instance H. Jones and N. F. Mott, Theory of the Properties of Metals and Alloys, Oxford University Press 1936). This “physicists view” takes the other extreme, where metallic systems are traditionally viewed as having no localized bonds at all, which leads to the so-called “band structure picture”. In vein the electrons are often described as being distributed throughout space in what is termed as a “free electron gas”. This so-called delocalized view of bonding versus the localized one still persists to this day and for small molecules chemists prefer to think of bonds as localized but for bulk metallic solids as delocalized. This becomes a conceptual problem in the nanoscale regime where the nanoparticles are usually too big to be thought of as molecules and to small to be thought of as solids. Ultimately, this difficulty leads to the question at what size regime does a particle become a metal?
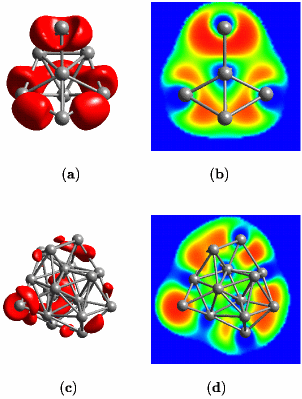
As a middle ground, one could conceive situations were one electron pair gets shared by more than two nuclei. This leads to the fruitful idea of “multi-center bonds”, the simplest one being the “three-center two-electron bond”; actually such a bond is operational in protonated methane. Given lithium, “the simplest metal” as an interesting example it was shown that the concept of multi-center bonds is useful in order to understand how chemical bonding evolves from the level of small insulating clusters to the metallic state and the surfaces of solids, see the left picture above. What is depicted on this webpage are two representations of the so-called electron localization function (ELF) introduced by Axel D. Becke and K. E. Edgecombe in The Journal of Chemical Physics 92, 5397 (1990). An oversimplified interpretation implies that “the amount of electron pairing” increases from blue to green to yellow to red according to the color-code. This function and its use in chemistry and physics is reviewed in Andreas Savin and coworkers, Angewandte Chemie Int. Ed. Engl. 36, 1808 (1997) or Angewandte Chemie 109, 1892 (1997) [in German] with lots of examples from organic, inorganic, and solid-state chemistry. A more basic exposition is presented in the article “Chemische Bindung anschaulich: die Elektronen-Lokalisierungs-Funktion - Die Pauli-Abstoßung sichtbar gemacht” by T. F. Fässler and A. Savin in Chemie in unserer Zeit 31, 110–120 (1997). What makes ELF useful in the present case is that it allows us to have a local view of chemical bonding even in metallic systems.
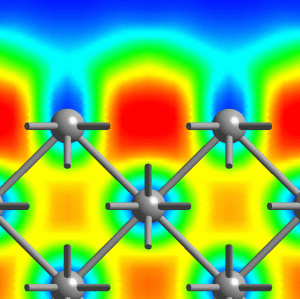
Small lithium clusters of up to 40 atoms are found to be dominated by multi-center bonds that involve three, four or even as much as six centers, see the middle figure above. It is exactly this pattern that finally transmutes into the “metallic bond” in the solid phase of lithium. Within this picture, the metallic phase of lithium is characterized by multi-center bonds and very gentle variations of ELF accross space. Most interesting, our results reveal that the core regions of quite small lithium clusters on the length scale of 1 nanometer (1 nm = 10-9m = 10Å) already exhibit bonding that is characteristic of bulk metallic solids, see panels (c) and (d) above and compare that to the left figure (cover page). However, the electrons on the outside of these nano-scale clusters behave like those of the small molecules. This allowed us to postulate and demonstrate that indeed a similar feature should exist on the surface of the bulk solid, see the right picture above (which shows a cut perpendicular to the (100)-surface of solid fcc lithium).
Another aspect to this work is the fact that unusually short Li–Li contacts were found in some clusters. It was shown that this strengthening is due to so-called p-pi interactions. They are directional and thus stabilize bonds only in certain directions. Most notably, there has been much excitement about these same short contacts which were found experimentally in solid lithium at exceedingly high pressures above 40 GPa, see Michael Hanfland and coworkers, “New high-pressure phases of lithium”, Nature 408 174 (2000). Theoretically it has even been postulated that such “Li–Li pairs” form the dominant feature of solid lithium at still higher compression (J. B. Neaton and N. W. Ashcroft, “Pairing in dense lithium”, Nature 400, 141 (1999)). Our analysis naturally explains these observations.
Finally, it is mentioned in passing that the electronic structure calculations that underlie the presented studies are based on the so-called Hohenberg-Kohn-Sham density functional theory, for the development of which Walter Kohn received the 1998 Nobel Price in Chemistry.
Further information and details:
- Calculations and analysis
- Roger Rousseau (Ruhr-Universität Bochum)
in collaboration with - Dominik Marx (Ruhr-Universität Bochum)
- Roger Rousseau (Ruhr-Universität Bochum)
- Scientific visualization
- Roger Rousseau (Ruhr-Universität Bochum)
- Additional information
- Chemical bonding in lithium: a unified view
R. Rousseau and D. Marx,
Exploring the Electronic Structure of Elemental Lithium: From Small Molecules to Nanoclusters, Bulk Metal, and Surfaces,
Chem. Eur. J. 6, 2982–2993 (2000); see also the Cover Page - Lithium clusters: structure and quantum effects
R. Rousseau and D. Marx,
The role of quantum and thermal fluctuations upon properties of lithium clusters,
J. Chem. Phys. 111, 5091–5101 (1999)
R. Rousseau and D. Marx,
Fluctuations and Bonding in Lithium Clusters,
Phys. Rev. Lett. 80, 2574–2577 (1998)
R. Rousseau and D. Marx,
Ab initio calculations on small lithium clusters,
Phys. Rev. A 56, 617–625 (1997) - Introduction into topological bifurcation analysis and ELF:
D. Marx and A. Savin,
Topological Bifurcation Analysis: Electronic Structure of CH5+,
Angew. Chem. Int. Ed. Engl. 36, 2077–2080 (1997) - Finally visit the magnificent home of ELF: http://www.cpfs.mpg.de/ELF/
- Chemical bonding in lithium: a unified view
- This web page
http://www.theochem.ruhr-uni-bochum.de/go/lithium.html